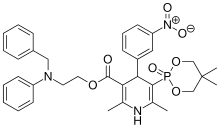
Efonidipine
- Molecular FormulaC34H38N3O7P
- Average mass631.655 Da
- エホニジピン
- CAS 111011-63-3; FREE FORM
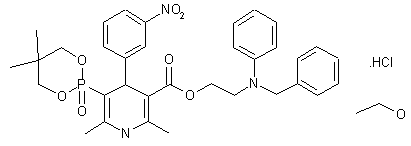

| Molecular Formula: | C36H45ClN3O8P |
|---|---|
| Molecular Weight: | 714.193 g/mol |
LD50:> 5 g/kg (R, p.o.)
- Synonyms:NZ-105
- ATC:C08CA
Efonidipine Hydrochloride Ethanolate Bulk & Tablets 10 mg/20mg/40mg, 

Efonidipine (INN) is a dihydropyridine calcium channel blocker marketed by Shionogi & Co. of Japan. It was launched in 1995, under the brand name Landel (ランデル). The drug blocks both T-type and L-type calcium channels.[1] Drug Controller General of India (DCGI) granted approval to M/s. Zuventus pharma Ltd for marketing efonidipine under brand name Efnocar in India .[2]
Structure Activity Relationship
Efonidipine is a dual Calcium Channel Blocker (L & T-type). It has a unique chemical structure. The phosphonate moiety (Figure 1) at the C5 position of the dihydropyridine ring is considered to be important for the characteristic pharmacological profile of the drug. (figure-1)
Mechanism of action
Efonidipine, a new generation dihydropyridine (DHP) calcium channel blocker, inhibits both L-type and T-type calcium channels.[1]
Pharmacodynamics
- Efonidipine exhibits antihypertensive effect through vasodilatation by blocking L-type and T-type calcium channels.[1]
- Efonidipine has a negative chronotropic effect. Working on sino atrial node cells by inhibiting T-type calcium channel activation, Efonidipine prolongs the late phase-4 depolarization of the sino atrial node action potential and suppresses an elevated HR. The negative chronotropic effect of Efonidipine decreases heart rate, myocardial oxygen demand and increases coronary blood flow.[3]
- Efonidipine increases coronary blood flow by blocking L & T-type calcium channels and attenuates myocardial ischaemia.[4]
- By reducing synthesis and secretion of aldosterone, Efonidipine prevents hypertrophy and remodeling of cardiac myocytes.[5]
- Efonidipine increases glomerular filtration rate without increasing intra-glomerular pressure and filtration fraction. This prevents hypertension induced renal damage.[6]
- Efonidipine prevents Rho-kinase and NFB induced renal parenchymal fibrosis and provides long term renal protection.[7][8]
- Efonidipine suppresses renin secretion from the juxta glomerular apparatus in the kidneys.[9]
- Efonidipine enhances sodium excretion from the kidneys by suppressing aldosterone synthesis and secretion from the adrenal glands. Aldosterone induced renal parenchymal fibrosis is suppressed by Efonidipine.[5]
- Efonidipine prevents NFB induced hypertrophy and inflammation in the renal vasculature and protects the kidneys.[7]
- Efonidipine protects against endothelial dysfunction due to its anti-oxidant activity and by restoring NO bioavailability.[10][11]
- Efonidipine has anti-atherogenic activity and protects the blood vessels from atherosclerosis.[12]
- Efonidipine lowers blood pressure in cerebral resistance vessels and prevents hypertension induced brain damage.[4]
Pharmacokinetics
Absorption
Peak plasma concentration is achieved in about 1.5 to 3.67 hours after administration. Half life is approximately 4 hours. The pharmacokinetic parameters of Efonidipine are depicted in Table-1.
Table 1: PK Parameters in Adult Healthy Male Subjects
| Variable | Efonidipine | |
| Mean | Range | |
| Cmax(ng/ml) | 36.25 | 9.66-66.91 |
| Tmax (hour) | 2.59 | 1.50-3.67 |
| T1/2 (hour) | 4.18 | 2.15-6.85 |
*Data on file
Long Duration of Action
Efonidipine has a slow onset and a long duration of action. This unique characteristic of Efonidipine is because of the following reasons:[13]
- High lipophilicity of Efonidipine allows it to enter the phospholipid rich cell membrane and access the dihydropyridine binding site of the Ca2+ channels.
- Tight binding to the dihydropyridine receptors.
- The dissociation constant of Efonidipine from dihydropyridine receptors is very low (0.0042/min/nM), signifying very slow dissociation from the receptors. This explains the long duration of action of Efonidipine.
Metabolism
Efonidipine is primarily metabolized in the liver. The important metabolites are N-dephenylated Efonidipine (DPH), deaminated Efonidipine (AL) and N-debenzylated Efonidipine (DBZ). DBZ and DPH exhibit activity as calcium antagonists. The vasodilating properties of DBZ and DPH were about two-thirds and one-third respectively than that of the parent compound. Results suggest that the majority of the pharmacological effect after oral dosing of Efonidipine hydrochloride in man is due to unchanged compound and its metabolites make a small contribution to the pharmacological effect.[14]
Elimination
Biliary route is the main pathway of excretion. No significant amount of unchanged drug was excreted in urine. In the urine collected for 24 h after an oral dosing, 1.1 % of the dose was excreted as deaminated Efonidipine, and 0.5% as a pyridine analogue of deaminated Efonidipine.
Indications
- Essential hypertension and renal parenchymal hypertension
- Angina
Dosage and Administration
- Essential hypertension and renal parenchymal hypertension: 20-40 mg orally once daily. A dose of up to 80mg/day is seen to be safe and effective in clinical trials.[15][16]
- Angina: 40 mg/day.
Contraindications
- Contraindicated in patients hypersensitive to Efonidipine or any of the excipients
- It is also contraindicated in pregnancy and lactation.
Precautions
- Should be administered with caution in patients with hepatic impairment
- Dose adjustment may be required in elderly as hypotension can occur
- Efonidipine may worsen clinical condition in patients with sinus bradycardia, sinus arrest or sinus node dysfunction
- As dizziness can occur due to hypotensive action, one should be careful while operating machines, with aerial work platforms and driving of a motor vehicle
- Drug should not be stopped abruptly. Discontinuation should be gradual and under supervision of a qualified physician
Drug Interactions
- Other anti-hypertensive agents: Efonidipine enhances the antihypertensive action additively and may produce hypotension and shock. Blood pressure should be monitored regularly to adjust dose of concomitant drugs.
- Cimetidine: Cimetidine inhibits CYP450 enzymes involved in metabolism of CCBs. Blood concentration of calcium channel antagonists increase leading to higher incidence of side effects (hot flushes).
- Grape fruit juice: Grapefruit juice suppresses enzymes metabolizing calcium channel antagonists (cytochrome P450) and reduces the clearance. Thus, there is a possibility that blood concentration of the drug may increase and the anti-hypertensive effect is enhanced.
- Tacrolimus: Efonidipine inhibits metabolic enzymes involved in Tacrolimus metabolism and reduces its clearance. So, increase in blood concentration of Tacrolimus can occur.
Adverse Drug Reactions
The common side effects are hot flushes, facial flushing and headache. In addition, elevation in serum total cholesterol, ALT (SGPT), AST (SGOT) and BUN may occur. Frequent urination, pedal edema, increased triglycerides occurs in less than 0.1%.[17]
Lesser incidence of pedal edema (< 0.1%)
One common adverse effect of the L-type Ca2+ channel blockers like Amlodipine is vasodilatory Pedal edema. Combined L-/T-type Ca2+ channel blockers, such as Efonidipine, display antihypertensive efficacy similar to their predecessors (Amlodipine) with much less propensity of pedal edema formation. Efonidipine equalizes the hydrostatic pressure across the capillary bed through equal arteriolar and venular dilatation, thus reducing vasodilatory edema. These incremental microcirculatory benefits of efonidipine over the conventional L-type Ca2+ channel blockers (Amlodipine) are likely attributed to their additional T-type Ca2+ channel blocking properties and the increased presence of T-type Ca2+channels in the microvasculature (e.g. arterioles, capillaries, venules etc).[18]
Among the CCBs, Efonidipine (<0.1%)[17] has lowest incidence of pedal edema compared to amlodipine ( 5-16%)[19], cilnidipine (5%)[20], benidipine (5%)[21] and azelnidipine (15.5%).[22]
Use in Special Population
Administration to Elderly
The drug should be started at low dose (20 mg/day) in elderly. Patient should be carefully observed for development of hypo-tension. Dose may be halved if there is intolerance to the 20 mg/day dosage regimen.
Pregnancy and Lactation
The drug should not be administered to pregnant women and women suspected of being pregnant. Administration to lactating women should be avoided unless benefit significantly surpasses the risk to the child. Mothers on Efonidipine treatment should avoid breast feeding.
Pediatric Use
Safety of Efonidipine in low birth weight infants, newborns, infants and children has not been established.
Efonidipine-The Best in Class
Efonidipine is unique among clinically available CCBs. Its antihypertensive efficacy is superior or at par with other CCBs. But, in terms of pleiotropic effects leading to enhanced cerebral, cardiac and renal protection, Efonidipine scores over the other CCBs.
Advantages over Amlodipine
1. Better renoprotection by:
- Dual channel blockade [1]
- Prevention of Rho-kinase and NFkB induced tubulointerstitial fibrosis[23][24]
- Reduction of synthesis and secretion of aldosterone from the adrenal cortex[25]
2. Preferred in angina with hypertension due to negative chronotropic action[26]
3. Better control of reflex tachycardia[3]
4. Reduces cardiac remodelling, arterial stiffness and prevents atherogenesis[27]
5. More useful in patients with diabetes & nephropathy[28]
6. Better protection against cardiac hypertrophy by significant reduction in LVMI[29]
7. Less adverse effects compared to Amlodipine[30]
8. Reduces endothelial dysfunction and oxidative stress(anti-oxidant property)[10]
Advantages over Cilnidipine
1. Strong negative chronotropic effect (less tachycardia) compared to Cilnidipine[3]
2. Significant improvement in exercise tolerance.[31]Better choice in hypertensive patients with angina.
3. Better BP control by marked urinary Na+ excretion[32]
4. Better renoprotection by:
- a. Suppression of plasma renin release[33]
- b. Prevention of Rho-kinase and NFkB induced tubulointerstitial fibrosis[34][35]
- c. Reduction of synthesis and secretion of aldosterone from the adrenal cortex[5]
5. Better choice in diabetic hypertensives[36]
6. Prevents cardiac remodelling by suppression of aldosterone secretion[5]
7. Superior anti-oxidant activity[10]
8. Less adverse effects compared to Cilnidipine[30]
Advantages over Benidipine
L & T-type CCBs have invoked a lot of interest in the management of hypertension because of their unique pharmacological profile. Several novel agents have been developed including Azelnidipine, Barnidipine, Benidipine, Efonidipine, Manidipine and Nilvadipine. Among all the agents, Efonidipine has emerged as the best among its peers. The advantages of Efonidipine over Benidipine are summarized below.
1. More selective blockade of T-type calcium channels [37][38]
2. More balanced renal arteriolar dilatation than benidipine[37][38]
3. Superior anti-proteinuric effect [15]
4. Greater reduction of serum aldosterone [39]
5. Renoprotection by reducing plasma renin unlike Benidipine [39]
6. Greater negative chronotropic effect
7. Efonidipine has anti-platelet activity[12]
8. Efonidipine reduces Insulin Resistance [40]
9. Significantly lower incidence of pedal edema & constipation compared to Benidipine

A new synthesis of efonidipine has been described: The cyclization of 2,2-dimethylbutane-1,4-diol (I) with triethyl phosphite (II) by heating at 100 C gives 2-methoxy-5,5-dimethyl-1,3,2-dioxaphosphorinan (III), which, by treatment with iodoacetone (IV) in refluxing ether, yields 2-acetonyl-5,5-dimethyl-1,3,2-dioxaphosphorinan-2-one (V). The condensation of (V) with 3-nitrobenzaldehyde (VI) by means of piperidine in acetic acid affords 3-(5,5-dimethyl-2-oxo-1,3,2-dioxaphosphorinan-2-yl)-4-(3-nitrophenyl)-3-buten-2-one (VII), which is finally cyclized with 3-amino-2-propenoic acid 2-(N-benzyl-N-phenylamino)ethyl ester (VIII) in refluxing toluene.ReferencesChem Pharm Bull 1992,40(9),2362

A new synthesis for (4S)-efonidipine has been described: The reaction of 5,5-dimethyl-2-(2-oxopropyl)-1,3,2-dioxaphosphorinan-2-one (I) with dimorpholino(3-nitrophenyl)methane (II) by means of trifluoroacetic acid in hot toluene gives 5,5-dimethyl-2-[1-acetyl-2-(3-nitrophenyl)vinyl]-1,3,2-dioxaphosphorina n-2-one (III), which is cyclized with 3-aminocrotonic acid 2(S)-methoxy-2-phenylethyl ester (IV) in refluxing toluene; the recrystallization of the resulting product affords 5-(5,5-dimethyl-2-oxo-1,3,2-dioxaphosphorinan-2-yl)-2,6-dimethyl-4(S)-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid 2(S)-methoxy-2-phenylethyl ester (V). The protection of the NH group of (V) with chloromethyl methyl ether and NaH in THF yields the N-methoxymethyl derivative (VI), which is transesterified with 2-(N-benzyl-N-methylamino)ethanol (VII) and NaH in DMSO, giving the protected final product (VIII). Finally, this compound is deprotected with HCl in ethanol.

An enantioselective synthesis of efonidipine has been described: The enantioselective hydrolysis of 5-(5,5-dimethyl-2-oxo-1,3,2-dioxaphosphorinan-2-yl)-2,6-dimethyl-4-(3-n itrophenyl)-1,4-dihydropyridine-3-carboxylic acid propionyloxymethyl ester (I) with lipase AH in 2,5-dimethyltetrahydrofuran saturated with water gives the corresponding free acid of the (S)-isomer (III), while the propionyloxymethyl ester of the (R)-isomer (II) remains undisturbed. After chromatographic separation, the (R)-ester (II) is hydrolyzed with NaOH in methanol to the (R)-acid (IV). Finally, both enantiomerically pure acids (III) and (IV) are separately esterified with 2-(N-benzyl-N-phenylamino)ethanol in the usual way
CLIP
PAPER
Synthesis of 1,4-dihydropyridine-5-phosphonates and their calcium antagonistic and antihypertensive activities: Novel calcium-antagonist 2-[benzyl(phenyl)amino]ethyl 5-(5,5-dimethyl-2-oxo-1,3,2-dioxaphosphorinan-2-yl)-1,4-dihydro-2, 6-dimethyl-4-(3-nitrophenyl)-3-pyridinecarboxylate hydrochloride ethanol (NZ-105) and its crystal structure
Chem Pharm Bull 1992, 40(9): 2362
PATENT
IN 201501586
http://ipindiaservices.gov.in/PatentSearch/PatentSearch/ViewPDF
- ^ Jump up to:a b c d Tanaka H, Shigenobu K (2002). “Efonidipine hydrochloride: a dual blocker of L- and T-type Ca2+ channels”. Cardiovasc. Drug Rev. 20 (1): 81–92. PMID 12070536.
- Jump up^http://www.cdsco.nic.in/writereaddata/Minutes%20of%2034th%20SEC%20Cardiovascular%20&%20Renal%2008_11_2016.pdf
- ^ Jump up to:a b c Masumiya H, Shijuku T, Tanaka H, Shigenobu K. Inhibition of myo¬cardial L- and T-type Ca2+ currents by efonidipine: possible mecha¬nism for its chronotropic effect. Eur J Pharmacol. 1998; 349: 351-7.
- ^ Jump up to:a b Masuda Y, Tanaka S. Efonidipine Hydrochloride: A New Calcium Antagonist. Cardiovascular Drug Reviews. 1994; 12 ( 2): 123-135.
- ^ Jump up to:a b c d Ikeda K, Isaka T, Fujioka K, Manome Y, Tojo K. Suppression of Aldosterone Synthesis and Secretion by Ca2+ Channel Antagonists. International Journal of Endocrinology. 2012.
- Jump up^ Hayashi K, et al. T-Type Ca Channel Blockade as a Determinant of Kidney Protection. Keio J Med. 2010; 59(3): 84-95
- ^ Jump up to:a b Hayashi M, Yamaji Y, Nakazato Y, Saruta T. The effects of calcium channel blockers on nuclear factor kappa B activation in the mesangial cells. Hypertens Res. 2000;23:521–525.
- Jump up^ Sugano N, Sugano N, Wakino S, Tatematsu S, Homma K, Yoshioka K, Hasegawa K, Utsunomiya Y, Tokudome G, Hosoya T, Saruta T, Hayashi K. Role of T-type Ca2 channels (TCCs) as a determinant of Rho-kinase activation and epithelial-mesenchymal transition (EMT) in renal injury. J Hypertens. 2006;24(suppl 6):128
- Jump up^ Baylis C, Qiu C, Engels K. Comparison of L-type and mixed L- and T-type calcium channel blockers on kidney injury caused by deoxycorticosterone-salt hypertension in rats. Am J Kidney Dis. 2001;38: 1292–1297.
- ^ Jump up to:a b c Sasaki H, Saiki A, Endo K, Ban N, Yamaguchi T, Kawana H, Nagayama D, Ohhira M, Oyama T, Miyashita Y, Shirai K. Protective effects of efonidipine, a T- and L-type calcium channel blocker, on renal function and arterial stiffness in type 2 diabetic patients with hypertension and nephropathy. J Atheroscler Thromb. 2009 Oct; 16(5): 568-75.
- Jump up^ Oshima T, Ozono R, Yano Y, Higashi Y, Teragawa H, Miho N, Ishida T, Ishida M, Yoshizumi M, Kambe M. Beneficial effect of T-type calcium channel blockers on endothelial function in patients with essential hypertension. Hypertens Res. 2005 Nov;28(11):889-94.
- ^ Jump up to:a b Nomura S, Kanazawa S, Fukuhara S. Effects of efonidipine on platelet and monocyte activation markers in hypertensive patients with and without type 2 diabetes mellitus. J Hum Hypertens. 2002 Aug;16(8):539-47.
- Jump up^ Yamashita T, Masuda Y, et al. NZ-105, a New 1,4-Dihydropyridine Derivative: Correlation between Dihydropyridine Receptor Binding and Inhibition of Calcium Uptake in Rabbit Aorta. Japan J Pharmacol. 1991; 57: 337-348.
- Jump up^ Nakabeppu H, et al.Metabolism of Efonidipine in Man.Xenobiotica.1995 Aug;229-239.
- ^ Jump up to:a b Hayashi K, Kumagai H, Saruta T. Effects of efonidipine and ACE inhibitors on proteinuria in human hypertension with renal impairment. Am J Hypertens. 2003;16:116 –122
- Jump up^ Oh IY, Seo MK, Lee HY, Kim SG, Kim KS, Kim WH, Hyon MS, Han KR, Lim SJ, Kim CH. Beneficial Effect of Efonidipine, an L- and T-Type Dual Calcium Channel Blocker, on Heart Rate and Blood Pressure in Patients With Mild-to-Moderate Essential Hypertension. Korean Circ J. 2010 Oct;40(10):514-9.
- ^ Jump up to:a b http://www.kegg.jp/medicus-bin/japic_med?japic_code=00044638. Missing or empty
|title=(help) - Jump up^ Ge W, Ren J. Combined L-/T-type calcium channel blockers: ready for prime time. Hypertension. 2009 Apr;53(4):592-4. doi: 10.1161/HYPERTENSIONAHA.108.127548. Epub 2009 Feb 23. PubMed PMID 19237678.
- Jump up^ Osterloh IH. An update on the safety of amlodipine. J Cardiovasc Pharmacol.1991;17 Suppl 1:S65-8.
- Jump up^ http://www.kegg.jp/medicus-bin/japic_med?japic_code=00062065. Missing or empty
|title=(help) - Jump up^ http://www.kegg.jp/medicus-bin/japic_med?japic_code=00005939. Missing or empty
|title=(help) - Jump up^ Takihata M, Nakamura A, Kondo Y, Kawasaki S, Kimura M, Terauchi Y. Comparison of Azelnidipine and Trichlormethiazide in Japanese Type 2 Diabetic Patients with Hypertension: The COAT Randomized Controlled Trial. PLoS One. 2015 May 4;10(5):e0125519.
- Jump up^ Song I, KimD, Choi S, Sun M, Kim Y, Shin HS. Role of the α1g T-type calcium channel in spontaneous absence seizures in mutant mice. J Neurosci. 2004; 24: 5249–5257.
- Jump up^ Lory P, Bidaud I, Chemin J. T-Type calcium channels in differentiation and proliferation. Cell Calcium. 2006; 40: 135–146.
- Jump up^ Ikeda K, Isaka T, Fujioka K, Manome Y, Tojo K. Suppression of Aldosterone Synthesis and Secretion by Ca2+ Channel Antagonists. International Journal of Endocrinology. 2012.
- Jump up^ Oh IY, Seo MK, Lee HY, Kim SG, Kim KS, Kim WH, Hyon MS, Han KR, Lim SJ, Kim CH. Beneficial Effect of Efonidipine, an L- and T-Type Dual Calcium Channel Blocker, on Heart Rate and Blood Pressure in Patients With Mild-to-Moderate Essential Hypertension. Korean Circ J. 2010 Oct;40(10):514-9.
- Jump up^ Catena C, Colussi G, Marzano L, Sechi LA. Aldosterone and the heart: from basic research to clinical evidence. Horm Metab Res. 2012;44:181– 187.
- Jump up^ Sasaki H, Saiki A, Endo K, Ban N, Yamaguchi T, Kawana H, Nagayama D, Ohhira M, Oyama T, Miyashita Y, Shirai K. Protective effects of efonidipine, a T- and L-type calcium channel blocker, on renal function and arterial stiffness in type 2 diabetic patients with hypertension and nephropathy. J Atheroscler Thromb. 2009 Oct; 16(5): 568-75.
- Jump up^ Saito T, Fujii K, Takizawa T, Toyosaki T, Kuwabara Y, Kobayashi S, Ichikawa H, Karaki A, Yamazaki Y, Iwata J, Yamada K, Tomiya H, Takeda K, Inagaki Y. Effects of the new calcium antagonist efonidipine hydrochloride on resting and exercise hemodynamics in patients with stable effort angina. Arzneimittelforschung. 1996 Sep;46(9):861-7.
- ^ Jump up to:a b Saruta T. Current status of calcium antagonists in Japan. Am J Cardiol. 1998;82:32R-34R.
- Jump up^ Okayama S, Imagawa K, Naya N, Iwama H, Somekawa S, Kawata H, Horii M, Nakajima T, Uemura S, Saito Y. Blocking T-type Ca2+ channels with efonidipine decreased plasma aldosterone concentration in healthy volunteers. Hypertens Res. 2006 Jul;29(7):493-7.
- Jump up^ Honda M, Hayashi K, Matsuda H, Kubota E, Tokuyama H, Okubo K, Ozawa Y, Saruta T. Divergent natriuretic action of calcium channel antagonists in mongrel dogs: renal haemodynamics as a determinant of natriuresis. Clinical Science. 2001; 101: 421–427
- Jump up^ Wagner C, Kramer KB, Hinder M, Kieninger M, Kurtz A. T-type and L-type calcium channel blockers exert opposite effects on renin secretion and renin gene expression in conscious rats. Br J Pharmacol. 1998;124: 579 –585.
- Jump up^ Song I, KimD, Choi S, Sun M, Kim Y, Shin HS. Role of the α1g T-type calcium channel in spontaneous absence seizures in mutant mice. J Neurosci. 2004; 24: 5249–5257.
- Jump up^ Lory P, Bidaud I, Chemin J. T-Type calcium channels in differentiation and proliferation. Cell Calcium. 2006; 40: 135–146.
- Jump up^ Ando K, Ueshima K, Tanaka S, Kosugi S, Sato T, Matsuoka H, Nakao K, Fujita T. Comparison of the antialbuminuric effects of L-/N-type and L-type calcium channel blockers in hypertensive patients with diabetes and microalbuminuria: the study of assessment for kidney function by urinary microalbumin in randomized (SAKURA) trial. Int J Med Sci. 2013 Jul 30;10(9):1209-16.
- ^ Jump up to:a b Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, Saruta T. Ca2+ Channel Subtypes and Pharmacology in the Kidney. Circ Res. 2007;100:342-353.
- ^ Jump up to:a b Hayashi K, Ozawa Y, Fujiwara K, Wakino S, Kumagai H, Saruta T. Role of actions of calcium antagonists on efferent arterioles with special references to glomerular hypertension. Am J Nephrol. 2003 Jul-Aug;23(4):229-44.
- ^ Jump up to:a b Tani S, Takahashi A, Nagao K, Hirayama A. Effects of the T/L-type calcium channel blocker benidipine on albuminuria and plasma aldosterone concentration. A pilot study involving switching from L-type calcium channel blockers to benidipine. Int Heart J. 2014;55(6):519-25
- Jump up^ Li M. Role of T-Type Ca2+ Channels in Basal Insulin Release. T-type Calcium Channels in Basic and Clinical Science. Springer Vienna. 2015; 137-150.
- (in Japanese) Landel ランデル (PDF) Shionogi & Co. April 2005.
 |
|
| Clinical data | |
|---|---|
| Trade names | Landel (ランデル) |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C34H38N3O7P |
| Molar mass | 631.65 g/mol |
| 3D model (JSmol) | |

(R)-base
- Formula:C34H38N3O7P
- MW:631.67 g/mol
- CAS-RN:128194-13-8
(S)-base
- Formula:C34H38N3O7P
- MW:631.67 g/mol
- CAS-RN:128194-12-7
///////////Efonidipine, エホニジピン, IND 2017, Landel , NZ 105, Efonidipine Hydrochloride Ethanolate
CC1=C(C(C(=C(N1)C)P2(=O)OCC(CO2)(C)C)C3=CC(=CC=C3)[N+](=O)[O-])C(=O)OCCN(CC4=CC=CC=C4)C5=CC=CC=C5



