Diazoxide choline,
RN: 1098065-76-9
UNII: 2U8NRZ7P8L
Diazoxide choline; UNII-2U8NRZ7P8L; 2U8NRZ7P8L; YLLWQNAEYILHLV-UHFFFAOYSA-N
| Molecular Formula: | C13H20ClN3O3S |
|---|---|
| Molecular Weight: | 333.831 g/mol |
Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, compd. with 7-chloro-3-methyl-2H-1,2,4-benzothiadiazine dioxide (1:1)
7-chloro-3-methyl-1$l^{6},2,4-benzothiadiazin-2-ide 1,1-dioxide;2-hydroxyethyl(trimethyl)azanium
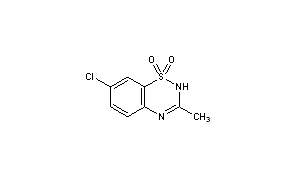 Diazoxide
Diazoxide
CAS: 364-98-7 FREE FORM
2H-1,2,4-Benzothiadiazine, 7-chloro-3-methyl-, 1,1-dioxide
- 4H-1,2,4-Benzothiadiazine, 7-chloro-3-methyl-, 1,1-dioxide (7CI)
- 3-Methyl-7-chloro-1,2,4-benzothiadiazine 1,1-dioxide
- 7-Chloro-3-methyl-2H-1,2,4-benzothiadiazine 1,1-dioxide
- Diazoxide
- Dizoxide
- Eudemine injection
- Hyperstat
- Hypertonalum
- Mutabase
- NSC 64198
- NSC 76130
- Proglicem
- Proglycem
- SRG 95213
- Sch 6783
Diazoxide (INN; brand name Proglycem[1]) is a potassium channel activator, which causes local relaxation in smooth muscle by increasing membrane permeability to potassium ions. This switches off voltage-gated calcium ion channels, preventing calcium flux across the sarcolemma and activation of the contractile apparatus.
In the United States, this agent is only available in the oral form and is typically given in hospital settings.[2]
Medical uses
Diazoxide is used as a vasodilator in the treatment of acute hypertension or malignant hypertension.[3]
Diazoxide also inhibits the secretion of insulin by opening ATP-sensitive potassium channel of beta cells of the pancreas, thus it is used to counter hypoglycemia in disease states such as insulinoma (a tumor producing insulin)[4] or congenital hyperinsulinism.
Diazoxide acts as a positive allosteric modulator of the AMPA and kainate receptors, suggesting potential application as a cognitive enhancer.[5]
Side effects
The Food and Drug Administration published a Safety Announcement in July 2015 highlighting the potential for development of pulmonary hypertension in newborns and infants treated with this drug.[2]Diazoxide interferes with insulin release through its action on potassium channels.[6] Diazoxide is one of the most potent openers of the K+ ATP channels present on the insulin producing beta cells of the pancreas. Opening these channels leads to hyperpolarization of cell membrane, a decrease in calcium influx, and a subsequently reduced release of insulin.[7] This mechanism of action is the mirror opposite of that of sulfonylureas, a class of medications used to increase insulin release in Type 2 Diabetics. Therefore, this medicine is not given to non-insulin dependent diabetic patients.
SYN
Medicinal Chemistry Research, 12(9), 457-470; 2004

PATENT
WO 2009006483
https://patents.google.com/patent/WO2009006483A1/enIt
PATENT
US 20120238554
PATENT
WO 2013130411
able 16. Characterization of Forms A and B of Diazoxide Choline Salt In
Screening Study
Experiment Form A Form B
*Maj or peaks (2-Θ):
Form A (9.8, 10.5, 14.9, 17.8, 17.9, 18.5, 19.5, 22.1, 22.6, 26.2, 29.6, 31.2);
Form B (8.9, 10.3, 12.0, 18.3, 20.6, 24.1, 24.5, 26.3, 27.1, 28.9).
** Unique FTIR (ATR) absorbances (cm 1):
Form A (2926, 2654, 1592, 1449, 1248);
Form B (3256, 2174, 2890, 1605, 1463, 1235).
6.1.5.1. Solubility Screen in organic solvents.
[00725] Diazoxide choline, prepared in MEK using choline hydroxide as 50 wt % solution in water (see above) displayed some solubility in the following solvents:
acetonitrile, acetone, ethanol, IPA, MEK, DMF, and methanol. These solvents were chosen due to differences in functionality, polarity, and boiling points and their ability to dissolve diazoxide. Other solvents which showed poor ability to dissolve salts were used as antisolvents and in slurry experiments where some solubility was observed: dioxane, MTBE, EtOAc, IP Ac, THF, water, cyclohexane, heptane, CH2C12, and toluene.
[00726] Solvents for crystallizations during screening were chosen based on the solubility screen summarized in Table 17. Crystallizations of diazoxide choline from all conditions afforded a total of two forms, A and B. Forms A and B were found to be anhydrous polymorphs of diazoxide choline. Form B was observed to be generated from most solvents used. It was difficult to isolate pure Form A on large scales (>50 mg) as conditions observed to produce Form A on a smaller scale (approximately 50 mg or less) were found to result in Form B or mixtures of both forms on larger scales. Based on room-temperature slurry experiments, anhydrous Form B was found to be the most thermodynamically stable form in this study. Form A readily converted to Form B in all slurry solvents utilized.
Table 17. Solubility Screen for Diazoxide Choline Salt
Solvent Cmpd Solvent Cone. Temp. Soluble
(mg) (mL) (mg/niL) (°C)
CH2CI2 1.3 5.00 0.26 55 Partially
Toluene 1.4 5.00 0.28 55 No
.1.5.2. Single-Solvent Crystallizations
[00727] Fast cooling procedure: Diazoxide (approximately 20 mg) was weighed out into vials and enough solvent (starting with 0.25 mL) was added until the material completely dissolved at elevated temperature. After hot filtration the vials were placed in a refrigerator (4 °C) for 16 hours. After the cooling-process the samples were observed for precipitates which were isolated by filtration. Vials not demonstrating precipitates were evaporated down to dryness using a gentle stream of nitrogen. All solids were dried in vacuo at ambient temperature and 30 in. Hg.
[00728] Slow cooling procedure: Diazoxide (approximately 30 mg of choline salt) was weighed out into vials and enough solvent was added until the material went into solution at elevated temperature. After hot filtration the vials were then slowly cooled to room temperature at the rate of 20 °C/h and stirred at room temperature for 1-2 hours. All solids were dried in vacuo at ambient temperature and 30 in. Hg.
[00729] Based on the initial solubility study, seven solvents were selected for the fast-cooling crystallization: acetonitrile, acetone, ethanol, IPA, MEK, DMF, and methanol. Table 18 shows a list of the solvents that were used and the amount of solvent needed to dissolve the material. After the cooling-process precipitates were noticed in samples # 2, 3, 5, and 6, the solids were isolated by filtration. The other samples (# 1, 4, and 7) were evaporated down to dryness using a gentle stream of nitrogen. The diazoxide choline salts were found to be consistent with Form A by XRPD analysis for all solids with the exception of sample #2 (consistent with the freeform) and sample #5 (consistent with Form B with preferred orientation observed).
Table 18. Single- Solvent Crystallization of Diazoxide Choline Salt Using Fast- Cooling Procedure
[00730] In accordance with the data obtained from fast-cooling experiments, four solvents which showed precipitation of solids were chosen for the slow-cooling experiments: MeOH, EtOH, MeCN, and IPA (Table 19). All obtained analyzable solids of the choline salt were found to be consistent with Form B by XRPD with the exception of Entry #1 which was consistent with diazoxide freeform and Entry #2 which was not analyzable. Mother liquor of Entry #2 was concentrated to dryness and the residual solids were analyzed by XRPD and found to be Form B material. As a result of obtaining freeform material from the single- solvent crystallizations in methanol, three more alcohols were tested for the single- solvent crystallizations using fast- and slow-cooling procedures. Tables 20 and 21 provide a list of the solvents that were used and the amount of solvent needed to dissolve the material. XRPD patterns of the fast-cooling procedure showed freeform of diazoxide from isobutanol, Form B from isoamyl alcohol, and Form A from tert-amyl alcohol compared to the slow-cooling procedure, which afforded Form B material from all three solvents.
Table 19. Single-Solvent Crystallization of Diazoxide Choline Salt Using Slow- Cooling Procedure
Table 20. Single- Solvent Crystallization of Diazoxide Choline Salt Using Fast- Cooling Procedure
Table 21. Single-Solvent Crystallization of Diazoxide Choline Salt Using Slow- Cooling Procedure
[00731] The results of the choline salt single- solvent fast- and slow-cooling crystallizations (see Tables 19 to 21) indicated that Form A was more likely to be isolated with fast-cooling profiles and Form B with slow-cooling profiles.
6.1.5.3. Binary Solvent Crystallizations
[00732] Binary- solvent crystallizations of the choline salt were performed using four primary solvents (MeOH, EtOH, IPA, and MeCN) and nine cosolvents (MTBE, EtOAc, IPAc, THF, c-hexane, heptane, toluene, CH2CI2, and dioxane) with a fast-cooling profile (supra). XRPD patterns showed that Form B was obtained from mixtures of MeOH with MTBE, EtOAc, IPAc, toluene, and dioxane. As shown in Table 22, Form A was obtained from mixtures of MeOH with THF and with CH2CI2 after evaporating the solvent to dryness. The mixtures of MeOH with cyclohexane and heptane provided the freeform of diazoxide. All solids obtained from fast-cooling procedures with EtOH, IPA, and MeCN as primary solvents provided Form B material.
Table 22. Binary-Solvent Crystallizations of Choline Salt of Diazoxide Using Fast- Cooling Procedure and MeOH as a Primary Solvent
* Solids were dissolved at 62 °C.
** Freeform of diazoxide.
[00733] Binary- solvent recrystallizations of the choline salt with the slow-cooling procedure were performed using two primary solvents (IPA and MeCN) and nine cosolvents (MTBE, EtOAc, IPAc, THF, c-hexane, heptane, toluene, CH2C12, and dioxane). All solids obtained from a slow-cooling procedure with IPA and MeCN as primary solvents provided Form B material based on XRPD analysis. The results of
binary- solvent crystallizations indicated that Form B was the most thermodynamic ally stable form of diazoxide choline.
6.1.5.4. Binary Solvent Crystallizations Using Water as a Cosolvent
[00734] In an attempt to investigate the formation of hydrates of the choline salt, experiments was performed using fast- and slow-cooling procedures and water as a cosolvent.
[00735] The fast cooling procedure (supra) was used with the exception of using different primary solvents which were miscible with water: acetone, acetonitrile, DMF, IPA, i-BuOH, i-AmOH, and t-AmOH. Water was utilized in these crystallizations as a cosolvent. All solids obtained from the fast-cooling procedure with water as the cosolvent provided diazoxide freeform material by XRPD analysis.
[00736] To compare the results obtained from the fast-cooling procedure a set of experiments was performed using a slow-cooling procedure and water as a cosolvent. All obtained solids were analyzed by XRPD and afforded patterns consistent with diazoxide freeform. Without wishing to be bound by theory, these results suggest that the conditions used for crystallization caused dissociation of the choline salt. A small amount of a second crop was obtained in each sample, but only two samples were analyzable by XRPD and indicated that the samples were freeform material. All mother liquors were evaporated to dryness and the residual solids were also analyzed by XRPD to afford patterns consistent with Form B of the choline salt.
6.1.5.5. Metastable Zone Width Estimation
[00737] Form B: To produce a robust process, an understanding of the solubility profiles of the various solid forms under consideration is required. From a practical standpoint, this involves the measurement of the metastable zone width (MSZW) of pure forms, whereby the saturation and supersaturation curves of the different forms are generated over a well defined concentration and temperature range. This knowledge can then be used to design a crystallization protocol that should ideally favor a selective crystal growth of the desired form.
[00738] Form B of diazoxide choline salt showed moderate solubility in a solvent mixture made of MeCN/MeOH/MtBE (10: 1: 12, volume ratios). The wide width of the metastable zone as shown in Table 23 gives many seeding options. During the MSZW measurement, aliquots from the crystallizing material were withdrawn and analyzed by XRPD to ensure that no form conversion occurred during the experiment. Indeed, the material remained unchanged during the test.
Table 23. Meta-Stable Zone Width For Form B Diazoxide Choline Salt in
MeCN/MeOH/MtBE (10:1:12) (v/v).
[00739] Form A: The metastable zone width for Form could not be estimated because this polymorphic form converted during the experiment to Form B.
6.1.5.6. Crystallization of Form A of Diazoxide Choline Salt
[00740] The choline salt of diazoxide (160.3 mg) was dissolved in 1 mL of IPA at 55 °C which was then passed through a Millipore 0.45 μΜ filter into a clean vial. This vial was placed in freezer a -20 °C overnight. Solids were not noticed and the flask was scratched with a micro- spatula. The vial was placed back in the freezer and nucleation was noticed after ten minutes. The solids were collected by vacuum filtration and washed with 1 mL of MtBE. The solids were dried in vacuo at 40 °C and 30 in. Hg to afford 70 mg (43.6% recovery) of Form A as determined by XRPD.
6.1.5.7. 500-mg Scale Crystallization of Form B of Diazoxide Choline Salt
[00741] The choline salt of diazoxide (524.3 mg) was dissolved in 3 mL of IPA at 78 °C and this solution was then cooled to 55 °C for the addition of MtBE. The MtBE (4 mL) was added until nucleation was observed. After nucleation the batch was allowed to cool to room temperature at a rate of 20 °C /h. The solids were collected by vacuum filtration and washed with 1 mL of MtBE. The solids were dried in vacuo at 40 °C and 30 in. of Hg to afford 426.7 mg (81.3% recovery) of Form B as determined by XRPD.
6.1.5.8. 2-g Scale Crystallization of Form B of Diazoxide Choline Salt
[00742] The choline salt of diazoxide (2.0015 g) was dissolved in 5.5 mL of IPA at 78 °C to afford a clear solution. This solution was passed through a Millipore Millex FH 0.45 μΜ filter. This solution was then cooled to 55 °C. MtBE was added in 1 mL portions, with a two minute interval between portions. Nucleation was noted after the second addition of MtBE. This suspension was allowed to cool to room temperature at a rate of 20 °C /h and stirred at this temperature for 16 hours. The solids were collected by vacuum filtration and washed with 1 mL of MtBE. The solids were dried in vacuo at 40 °C and 30 in. of Hg to afford 1.6091 g (80.4% recovery) of Form B as determined by XRPD.
6.1.5.9. Detection of Form Impurities
[00743] Mixtures of diazoxide choline Forms A and B were prepared by adding a minor amount of Form A to Form B. Samples were lightly ground by hands with a mortar and pestle for approximately one minute. Samples were then analyzed by XRPD analysis. XRPD analysis was found to be suitable for detecting 5% of Form A in Form B.
References
- Jump up^ Diazoxide, drugs.com
- ^ Jump up to:a b “FDA Drug Safety Communication: FDA warns about a serious lung condition in infants and newborns treated with Proglycem (diazoxide)” (Press release). Food and Drug Administration. July 16, 2015. Retrieved 2015-07-19.
- Jump up^ van Hamersvelt HW, Kloke HJ, de Jong DJ, Koene RA, Huysmans FT (August 1996). “Oedema formation with the vasodilators nifedipine and diazoxide: direct local effect or sodium retention?”. Journal of Hypertension. 14 (8): 1041–5. doi:10.1097/00004872-199608000-00016. PMID 8884561.
![closed access publication – behind paywall]()
- Jump up^ Huang Q, Bu S, Yu Y, et al. (January 2007). “Diazoxide prevents diabetes through inhibiting pancreatic beta-cells from apoptosis via Bcl-2/Bax rate and p38-beta mitogen-activated protein kinase”. Endocrinology. 148 (1): 81–91. doi:10.1210/en.2006-0738. PMID 17053028.
![open access publication – free to read]()
- Jump up^ Randle, John C.R.; Biton, Catherine; Lepagnol, Jean M. (15 November 1993). “Allosteric potentiation by diazoxide of AMPA receptor currents and synaptic potentials”. European Journal of Pharmacology. 247 (3): 257–65. doi:10.1016/0922-4106(93)90193-D. PMID 8307099.
![closed access publication – behind paywall]()
- Jump up^ Panten, Uwe; Burgfeld, Johanna; Goerke, Frank; Rennicke, Michael; Schwanstecher, Mathias; Wallasch, Andreas; Zünkler, Bernd J.; Lenzen, Sigurd (1989-04-15). “Control of insulin secretion by sulfonylureas, meglitinide and diazoxide in relation to their binding to the sulfonylurea receptor in pancreatic islets”. Biochemical Pharmacology. 38 (8): 1217–1229. doi:10.1016/0006-2952(89)90327-4.
- Jump up^ Doyle, Máire E.; Egan, Josephine M. (2003-03-01). “Pharmacological Agents That Directly Modulate Insulin Secretion”. Pharmacological Reviews. 55 (1): 105–131. doi:10.1124/pr.55.1.7. ISSN 1521-0081. PMID 12615955.
 |
|
| Clinical data | |
|---|---|
| Trade names | Proglycem |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration |
Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 90% |
| Metabolism | Hepatic oxidation and sulfate conjugation |
| Elimination half-life | 21-45 hours |
| Excretion | Renal |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.006.063  |
| Chemical and physical data | |
| Formula | C8H7ClN2O2S |
| Molar mass | 230.672 g/mol |
| 3D model (JSmol) | |
//////////////Diazoxide choline
CC1=NC2=C(C=C(C=C2)Cl)S(=O)(=O)[N-]1.C[N+](C)(C)CCO
CC1=NC2=C(C=C(C=C2)Cl)S(=O)(=O)[N-]1.C[N+](C)(C)CCO



