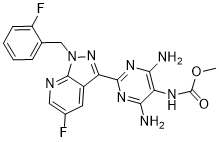
Vericiguat
BAY 102; BAY-1021189; MK-1242
1350653-20-1
Chemical Formula: C19H16F2N8O2
Molecular Weight: 426.3878
Vericiguat; 1350653-20-1; UNII-LV66ADM269; Methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-yl)carbamate; BAY-1021189; LV66ADM269
Methyl (4,6-diamino-2-(5-fluoro-1-((2-fluorophenyl)methyl)-1H-pyrazolo(3,4-b)pyridin-3-yl(pyrimidin-5-yl)carbamate
methyl N-[4,6-diamino-2-[5-fluoro-1-[(2-fluorophenyl)methyl]pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]carbamate
Methyl{4,6-diamino-2-[5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridi- n-3-yl]pyrimidin-5-yl}carbamate
- Originator Bayer HealthCare Pharmaceuticals
- Developer Bayer HealthCare Pharmaceuticals; Merck & Co
- Mechanism of Action Guanylate cyclase stimulants
- Phase III Chronic heart failure
- Phase I Coronary artery disease
- 28 May 2018 Phase II VITALITY HFpEF trial for Chronic heart failure in Austria, USA, Belgium, Portugal, Canada, Spain, Hungary and Greece (PO) (EudraCT2018-000298-65) (NCT03547583)
- 17 May 2018 Phase-I clinical trials in Coronary artery disease (In adults, In the elderly) in Moldova and Germany (PO) (NCT03504982)
- 20 Apr 2018 Bayer in collaboration with Merck Sharp & Dohme Corp. plans a phase I trial for Coronary Artery Disease in the Netherlands, Moldova and Germany (NCT03504982)
Vericiguat, also known as BAY1021189 or BAY10-21189, is a potent and orally active sGC stimulator (Soluble Guanylate Cyclase Stimulator). Direct stimulation of soluble guanylate cyclase (sGC) is emerging as a potential new approach for the treatment of renal disorders. sGC catalyzes the formation of cyclic guanosine monophosphate (cGMP), deficiency of which is implicated in the pathogenesis of chronic kidney disease (CKD).
Vericiguat, discovered at Bayer, is the first soluble guanylate cyclase (sGC) stimulator. Vericiguat is currently being studied in a Phase III clinical program for the treatment of heart failure with reduced ejection fraction (HFrEF)
| ベルイシグアト Vericiguat  C19H16F2N8O2 : 426.38 [1350653-20-1] |
Vericiguat hydrochloride
cas 1350658-96-6
PHASE 3 MERCK/BAYER
| Chemical Names: | UNII-5G76IGF54K; 5G76IGF54K; ; 1350658-96-6; Carbamic acid, N-(4,6-diamino-2-(5-fluoro-1-((2-fluorophenyl)methyl)-1H-pyrazolo(3,4-b)pyridin-3-yl)-5-pyrimidinyl)-, methyl ester, hydrochloride (1:1); Methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo(3,4-b)pyridin-3-yl)pyrimidin-5-yl)carbamate hydrochloride |
|---|---|
| Molecular Formula: | C19H17ClF2N8O2 |
| Molecular Weight: | 462.846 g/mol |

Clip
https://www.thieme-connect.com/products/ejournals/pdf/10.1055/s-0036-1590758.pdf

Significance: Vericiguat (BAY 1021189) is an orally available soluble guanylate cyclase (sGC) stimulator that has entered phase-three trials for the once-daily treatment of chronic heart failure. Key steps in the synthesis depicted are (1) construction of the 5-fluoro-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxylate C by condensation of the 5-amino-1H-pyrazole-3-carboxylate A with the aldehyde B and (2) construction of the pyrimidine-4,5,6-triamine derivative H through reaction of [(E)-phenyldiazenyl]malononitrile (G) with amidine F.
Comment: Experimental details are provided for the noteworthy four-step synthesis (not shown) of the crystalline 2-fluoro-(3-morpholin-4-yl)acrylaldehyde B from commercially available 2,2,3,3- tetrafluoro-1-propanol. The synthesis of pyrazole A is described in a patent (A. Straub et al. WO 2000/006569 A1). The [(E)-phenyldiazenyl]malononitrile (G) was generated in situ by reaction of phenyldiazonium chloride with malononitrile.
REFERENCES
1: Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET, Müller K, Roessig L, Pieske B; SOCRATES-REDUCED Investigators and Coordinators. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA. 2015 Dec 1;314(21):2251-62. doi: 10.1001/jama.2015.15734. PubMed PMID: 26547357.
2: Tschöpe C, Pieske B. [New therapy concepts for heart failure with preserved ejection fraction]. Herz. 2015 Apr;40(2):194-205. doi: 10.1007/s00059-015-4210-x. German. PubMed PMID: 25737289.
3: Stasch JP, Schlossmann J, Hocher B. Renal effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Curr Opin Pharmacol. 2015 Apr;21:95-104. doi: 10.1016/j.coph.2014.12.014. Epub 2015 Jan 31. Review. PubMed PMID: 25645316.
4: Pieske B, Butler J, Filippatos G, Lam C, Maggioni AP, Ponikowski P, Shah S, Solomon S, Kraigher-Krainer E, Samano ET, Scalise AV, Müller K, Roessig L, Gheorghiade M; SOCRATES Investigators and Coordinators. Rationale and design of the SOluble guanylate Cyclase stimulatoR in heArT failurE Studies (SOCRATES). Eur J Heart Fail. 2014 Sep;16(9):1026-38. doi: 10.1002/ejhf.135. Epub 2014 Jul 24. PubMed PMID: 25056511.
5: Stasch JP, Evgenov OV. Soluble guanylate cyclase stimulators in pulmonary hypertension. Handb Exp Pharmacol. 2013;218:279-313. doi: 10.1007/978-3-642-38664-0_12. Review. PubMed PMID: 24092345.
////////////////Vericiguat, BAY 102, BAY-1021189, MK-1242, ベルイシグアト , PHASE 3, MERCK, BAYER
COC(=O)NC1=C(N=C(N=C1N)C2=NN(C3=NC=C(C=C23)F)CC4=CC=CC=C4F)N

