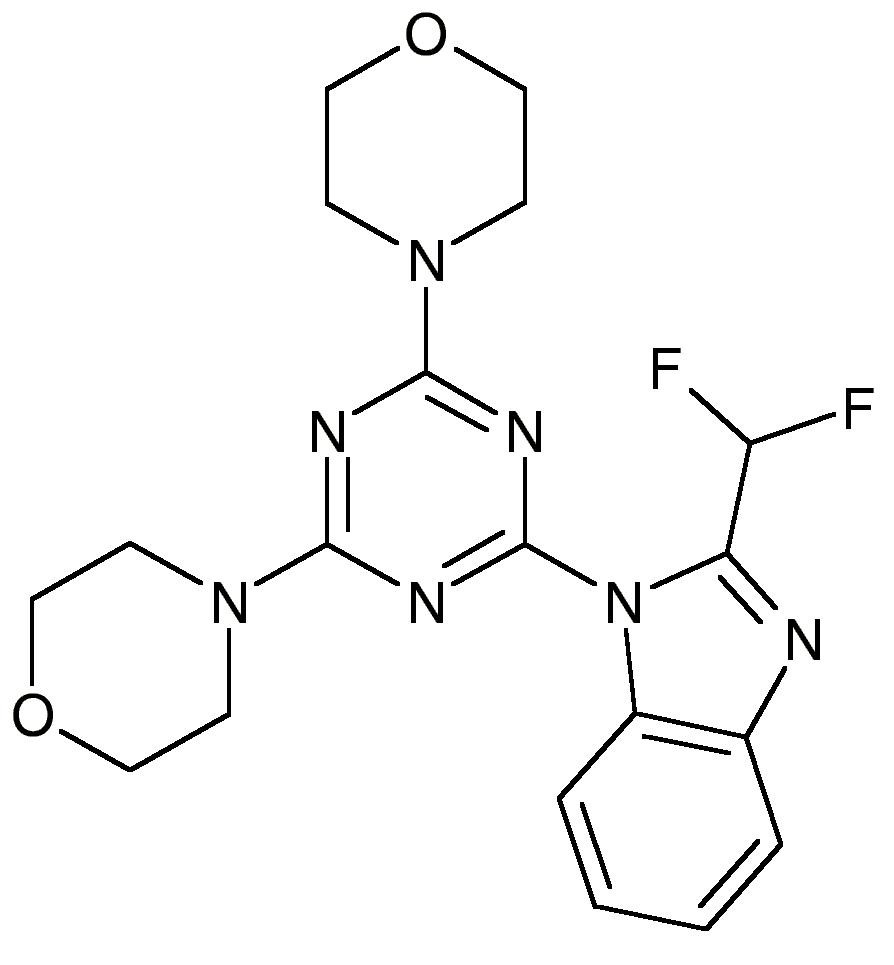
4-[4-[2-(difluoromethyl)benzimidazol-1-yl]-6-morpholin-4-yl-1,3,5-triazin-2-yl]morpholine
ZSTK474; 475110-96-4; 4,4′-(6-(2-(Difluoromethyl)-1H-benzo[d]imidazol-1-yl)-1,3,5-triazine-2,4-diyl)dimorpholine; ZSTK-474; ZSTK 474; TCMDC-137004;
2-(2-Difluoromethylbenzimidazol-1-yl)-4,6-bis(morpholino)-1,3,5-triazine
2-(2-difluoromethylbenzimidazol-1-yl)-4,6-dimorpholino-1,3,5-triazine
Zenyaku Kogyo (Innovator)
phase2………Treatment of Solid Tumors Therapy
ZSTK474 is a cell permeable and reversible P13K inhibitor with an IC₅₀ at 6nm. It was identified as part of a screening library, selected for its ability to block tumor cell growth. ZSTK474 has shown strong antitumor activities against human cancer xenographs when administered orally to mice without a significant toxic effect.
Phosphatidylinositol 3-kinase (PI3K) has been implicated in a variety of diseases including cancer. A number of PI3K inhibitors have recently been developed for use in cancer therapy. ZSTK474 is a highly promising antitumor agent targeting PI3K. We previously reported that ZSTK474 showed potent inhibition against four class I PI3K isoforms but not against 140 protein kinases.
However, whether ZSTK474 inhibits DNA-dependent protein kinase (DNA-PK), which is structurally similar to PI3K, remains unknown. To investigate the inhibition of DNA-PK, we developed a new DNA-PK assay method using Kinase-Glo. The inhibition activity of ZSTK474 against DNA-PK was determined, and shown to be far weaker compared with that observed against PI3K. The inhibition selectivity of ZSTK474 for PI3K over DNA-PK was significantly higher than other PI3K inhibitors, namely NVP-BEZ235, PI-103 and LY294002.
Other Names: ZSTK-474
Chemical Formula: C19H21F2N7O2
CAS Number: 475110-96-4
Molecular Weight: 417.41
WO 2002088112
http://www.google.co.in/patents/EP1389617A1?cl=en
The condensation of 2,4-dichloro-6-(4-morpholinyl)-1,3,5-triazine

with 2-(difluoromethyl)-1H-benzimidazole by means of K2CO3 in DMF gives

2-chloro-4-[2-(difluoromethyl)-1H-benzimidazol-1-yl]-6-(4-morpholinyl)-1,3,5-triazine ,

which is then condensed with morpholine by means of K2CO3 in DMF to afford the target trisubstituted triazine.

aReagents and conditions: (i) K2CO3, DMF, room temp; (ii) morpholine, DMF or THF, room temp; (iii) NaH or K2CO3, DMF or DMSO, 120 °C.

- 2-(2-difluoromethylbenzimidazol-1-yl)-4,6-dimorpholino-1,3,5-triazine(compound 19)
Melting point: 211-214°C
NMR(CDCl3) δ : 3.79(8H, t, J=4Hz), 3.88(8H, t, J=4Hz), 7.3-7.4(2H, m), 7.56(1H, t, J=53Hz), 7.88(1H, d, J=7Hz), 8.32(1H, d, J=7Hz)
MS m/z: 417(M+
……………………
1H NMR (CDCl3) δ 8.33 (dd, J = 7.3, 1.4 Hz, 1H), 7.89 (dd, J = 7.2, 1.5 Hz, 1H), 7.56 (t, JHF= 53.6 Hz, 1H), 7.46–7.37 (m, 2H), 3.91–3.86 (m, 8H), 3.81–3.76 (m, 8H).

TRIAZINE, PYRIMIDINE AND PYRIDINE ANALOGS AND THEIR USE AS THERAPEUTIC AGENTS AND DIAGNOSTIC PROBES [US2011275762]2011-11-10
| Patent | Submitted | Granted |
|---|---|---|
| Heterocyclic compound and antitumor agent containing the same as active ingredient [US7071189] | 2004-06-17 | 2006-07-04 |
| Treatment of prostate cancer, melanoma or hepatic cancer [US2007244110] | 2007-10-18 | |
| Heterocyclic compound and antitumor agent containing the same as effective ingredient [US7307077] | 2006-11-02 | 2007-12-11 |
| IMMUNOSUPPRESSIVE AGENT AND ANTI-TUMOR AGENT COMPRISING HETEROCYCLIC COMPOUND AS ACTIVE INGREDIENT [US7750001] | 2008-05-15 | 2010-07-06 |
| PYRIMIDINYL AND 1,3,5-TRIAZINYL BENZIMIDAZOLES AND THEIR USE IN CANCER THERAPY [US2011009405] | 2011-01-13 | |
| SUBSTITUTED PYRIMIDINES AND TRIAZINES AND THEIR USE IN CANCER THERAPY [US2011053907] | 2011-03-03 | |
| IMMUNOSUPPRESSIVE AGENT AND ANTI-TUMOR AGENT COMPRISING HETEROCYCLIC COMPOUND AS ACTIVE INGREDIENT [US2010267700] | 2010-10-21 | |
| AMORPHOUS BODY COMPOSED OF HETEROCYCLIC COMPOUND, SOLID DISPERSION AND PHARMACEUTICAL PREPARATION EACH COMPRISING THE SAME, AND PROCESS FOR PRODUCTION OF THE SAME [US8227463] | 2010-09-30 | 2012-07-24 |
| PYRAZOLO[1,5-a]PYRIDINES AND THEIR USE IN CANCER THERAPY [US2010226881] | 2010-09-09 | |
| PYRIMIDINYL AND 1,3,5-TRIAZINYL BENZIMIDAZOLE SULFONAMIDES AND THEIR USE IN CANCER THERAPY [US2010249099] | 2010-09-30 |
…………..
Zenyaku Kogyo





Filed under: cancer, Uncategorized Tagged: Bunkyo, CANCER, cancer therapy, DNA-PK, Heterocyclic compound, otsuka, phase 2, Zenyaku Kogyo, ZSTK 474, ZSTK474
